Startup looks to sell IV product in South Korea
by May 27, 2019 12:27 pm 476 views
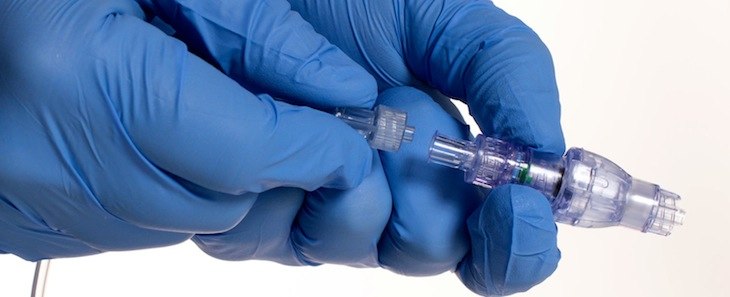
Healthcare startup Lineus Medical of Fayetteville looks to receive clearance to sell its IV device in South Korea, where IV usage is twice per capita of the United States.
The startup is working with the Korean Ministry of Food and Drug Safety to receive the clearance for SafeBreak Vascular and to establish a distribution network in Korea, said Vance Clement, CEO of Lineus Medical. SafeBreak Vascular is the company’s first product, and it’s expected to help prevent IV lines from failing.
“South Korea has a population of over 50 million, and IV usage is twice per capita of the United States,” Clement said. “We intend to start selling SafeBreak in Korea as our first Asian market and use it as a springboard into other Asian countries.”
Lineus Medical recently placed fourth in the K-Startup Grand Challenge, sponsored by the Korean Government. The competition took place in Seoul, Korea, from August to December 2018, and Lineus Medical was one of 80 teams initially selected for the competition before becoming a top 40 team in early December. The K-Startup Challenge is the largest business accelerator for companies looking to enter the Korean/Asian market, according to a news release.
Louis Diesel, director of business development in Asia for Lineus Medical, was the company’s primary representative in the competition. “I couldn’t be happier with our finish in the K-Startup Challenge,” he said. “It was a privilege to be chosen and to participate in the program. We could never have obtained the support, guidance and opportunities on our own. I look forward to gaining regulatory clearance in Korea and starting to make an impact on saving IV lines in Asia.”
The SafeBreak Vascular device attaches to an IV line. When force is applied to the line, the two pieces break away from each other, creating “a controlled separation,” preventing damage to the IV line and not requiring the insertion of a new one, according to the company’s website. The device has yet to receive clearance from the U.S. Food and Drug Administration.
