Springdale-based startup ready for multi-state test of advanced breast cancer detection system
by October 19, 2016 7:38 am 1,604 views
A clinician takes a sample of tears from a patient’s tear duct which is then tested for breast cancer with a patent-pending detection system owned by Springdale-based Ascendant Dx.
Scientists at Ascendant Dx hope to raise enough money to launch a multi-state clinical trial of what they believe is a groundbreaking process that changes how – and more importantly, when – breast cancer is diagnosed in women.
Dr. Suzanne Klimberg, a surgeon at University of Arkansas for Medical Sciences in Little Rock, discovered breast cancer proteins that are visible in tears about 10 years ago. That discovery led her to co-invent a process and device known as MelodyDx which can detect breast cancer in woman by collecting their tears.
Using filter paper placed under the eyelid, tears are absorbed by the paper. Proteins from the tears are then identified in less than 30 minutes in the patent-pending MelodyDx kit, much like home pregnancy test, according to lead researcher Dr. Anna Daily who has continued to develop the process since 2011.
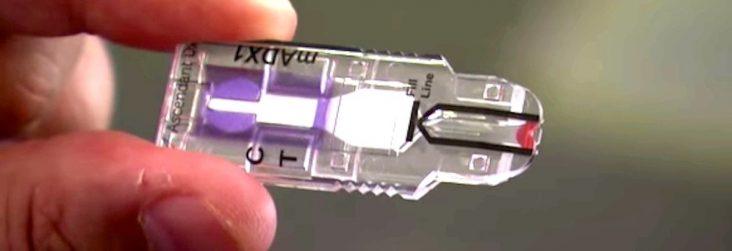
The MelodyDx device resembles a memory stick and once the proteins react with the cartridge a line appears if there is positive reading for breast cancer. The device itself is far less expensive than mammography and has a sensitivity rate of 90%, nearly twice as accurate as mammograms at finding early cancer, according to Ascendant.
Springdale-based Ascendant Dx was birthed in the University of Arkansas’ technology park in Fayetteville and in early 2014 obtained $2 million in an investment round to help with the clinical trial and commercialization push.
CHANGING VIEWS ON BREAST CANCER DETECTION
Molecular biologist Daily told Talk Business & Politics she is one of two full-time scientists working on the application at Ascendant. She said the proteins found in tears are much clearer than those found in blood or urine. She said test trials conducted in Northwest Arkansas earlier this year found tear duct secretions were able to be used to detect even the earliest of breast cancer. She said the screening method has proven to be sensitive finding cancer about 90% of time, better than an average 60% sensitivity rate for mammography.
Dr. Steven Harms, a radiologist at the Breast Center in Fayetteville, said had he not been part of the study and seen patients benefit from this early detection, he might not have believed it.
“Frankly it just sounded to good to be true,” Harms said. “Taking part in this study and seeing the tear duct screening results signal very early cancers has changed my own views about breast cancer. I used to think that cancer could sit silent in the breast for sometime before the rest of the body knew it was there. But this test showed me that even the very earliest point of cancer in the breast there are transmitters in the body taking note with protein secretions. This is huge and has the potential to change the way screening is done around the world.”
He said mammography has been the standard method for screening for years. And even though it’s widely covered by insurance, and Arkansas has funding for uninsured women to have the test, he said only about 50% of women have an annual screening. In some counties in Arkansas he said the percentage of woman getting annual mammograms is as low as 25%.
The MelodyDx test could be viable alternative in areas where access to mammography is limited and it could also be used as a screening application for all women, including younger women who often don’t have mammograms. Daily said there are no plans to develop an over-the-counter kit because the test done should be conducted in a controlled, clinical setting. She said perhaps it could be part of annual wellness checkup just like a pap smear which detects cervical cancer.
BREAST CANCER ‘CURABLE’ IF FOUND EARLY
Harms said the cure for breast cancer is early detection and the best screening methods are the way to make that happen more often.
“If breast cancer was found while it was less than 1 centimeter in size it’s virtually curable today. That means very early detection is required. The MelodyDx could change the paradigm for how we screen for cancer in the future. This inexpensive test can be widely done at low costs, allowing us to use the more expensive screenings like MRI on only those few positive readings,” Harms said.
He said mammograms are only half as sensitive in finding cancer in women with dense breast tissue, and MelodyDx would be used for those women. Those with positive findings could have the more expensive MRI which has the highest level of detectability.
This year more than 246,000 women will be diagnosed with invasive breast cancer and another 61,000 will have in-situ or non-invasive cancer, according to Breastcancer.org. About 40,450 women in the U.S. are expected to die in 2016 from breast cancer, though death rates have been decreasing since 1989.
Outside the U.S., death rates from breast cancer are as high as 58% in some parts of Africa, Harms said the new MelodyDx testing could radically reduce deaths from cancer in all four corners of the earth.
“I am proud to see Arkansas develop something like this within our research hospital (UAMS) and then incubate and grow the opportunity at the UA. This is a major home run and many people have poured themselves into furthering this effort,” Harms said in a statement. “This doesn’t happen very often anywhere, including at Harvard or at Stanford and to think it came from Arkansas is big deal.”
BROADER TESTING REQUIRED
Daily said plans are for the startup to test the MelodyDx screening process and device in a multi-state, long-term trial that would cover Arkansas, Texas and Oklahoma with some additional exposure in the Pacific Northwest – primarily in Alaska, Oregon and Washington through a partnership with PeaceHealth Laboratories. She said the long term trials are required if the process is get FDA approval in the future.
As with any startup, raising capital is key to funding its existence. Daily said Ascendant Dx has subsisted on angel funding and other private investments thus far. The company is guarded about fundraising numbers, and Ascendant Dx CEO Omid Moghadam sent this statement to Talk Business & Politics when asked about fundraising: “Due to the rules surrounding fundraising for a company of this size, they cannot make any comments around fundraising. If anyone would like further information they are welcome to reach out to the company directly.”
However, cost estimates for conducting multi-state trials range between $4 million and $6 million to start.
Moghadam in August presented MelodyDx at the prestigious South by Southwest startup conference in Austin, Texas, and in October he was part of the South by Southwest Lawn technology conference at the White House. Moghadam, a former Harvard researcher and entrepreneur, was one of eight startup execs selected for the Austin lineup focused on commercializing disruptive technologies with social implications. SXSW is an opportunity for startups to pitch their technologies to investors.
