Fayetteville startup completes $3M Series B funding round; ready for product launch
by August 30, 2021 8:03 am 1,307 views
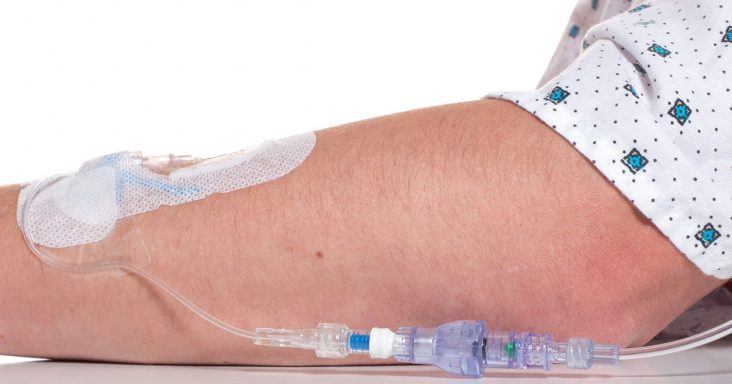
A SafeBreak Vascular installed in an IV line.
Fayetteville-based healthcare technology startup Lineus Medical announced Monday (Aug. 30) the completion of a $3 million Series B funding round. The second tranche of $1.75 million recently came through. The first tranche of $1.25 million was completed in March 2020.
According to a news release, Lineus Medical has raised $6.4 million to support the development and launch of the company’s first medical device, SafeBreak Vascular. Founder and CTO Spencer Jones founded Lineus Medical, formerly LineGard Med, in May 2015 to develop and bring SafeBreak Vascular to market.
KMF Investments of Denton, Texas, led the second tranche of funding. According to the release, KMF will have a seat on the Lineus Medical board of directors. Another key investor in the round is Hartford HealthCare Corp., which owns Hartford Hospital in Hartford, Conn., that conducted a key clinical study on SafeBreak Vascular.
“We are excited to see the progress Lineus Medical has made in the past couple of years: completing compelling clinical trials, building a strong team and securing regulatory clearance. We are pleased to lead this funding round to support the launch of SafeBreak,” said Jonathon Fite, managing partner from KMF Investments.
SafeBreak Vascular is a break-away connector that fits into any peripheral IV line anywhere in the world, although currently it is only cleared for use in the United States. The device is designed to separate when a damaging force is placed on an IV line, therefore reducing IV site irritation and the chances of the IV dislodging. When the device separates, valves on each side of the device close to stop the flow of medication from the pump and blood flow from the patient’s IV catheter. The separated SafeBreak can be replaced with a new, sterile SafeBreak and the IV restarted without the need for another needlestick for the patient.
Lineus Medical was cleared by the FDA in May to begin selling the SafeBreak Vascular product.
“Additionally, in January, we completed a 303-person randomized controlled trial showing SafeBreak Vascular reduces peripheral IV complications requiring an IV restart by 44%,” CEO Vance Clement said in a statement. “We have FDA clearance, we have a product that has been shown to make a huge impact in the hospital, and now we have the funding needed to launch the product and drive adoption.”
IV restarts are estimated to cost the U.S. healthcare system more than $5 billion annually. The cost of a restart is about $50 each. More than 235 million IV lines are placed annually in the United States, and an average of 46% fail before their intended end of use, the release shows.
