Lineus Medical awarded $100K in TDP funding from the Arkansas Economic Development Commission
by August 23, 2021 9:18 am 1,008 views
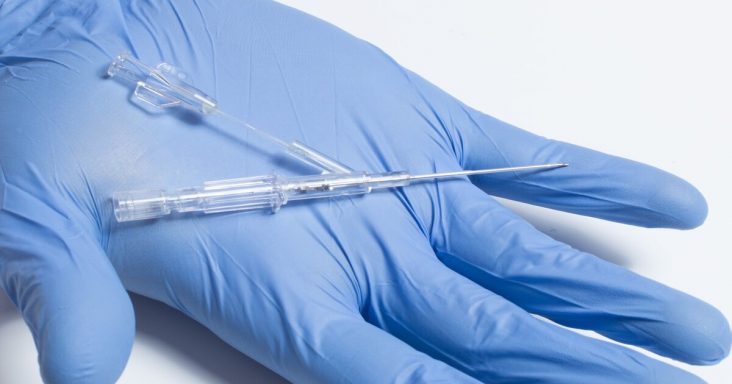
Lineus Medical's BVAD (Bifurcated Venous Access Device).
The Arkansas Economic Development Commission’s Technology Development Program (TDP) has awarded $100,000 to healthcare startup Lineus Medical of Fayetteville.
The TDP program helps develop new technology-based products and processes through royalty-based financing for qualified science and technology projects with a potential for economic and employment growth in Arkansas.
In a news release Monday (Aug. 23), Lineus Medical officials say the funding will be used to further develop BVAD (Bifurcated Venous Access Device), Lineus Medical’s patented, dual lumen, peripheral IV catheter.
According to the release, this is the first time Lineus Medical has received TDP funding from the AEDC.
“The design enhancement we’re investigating has the potential to reduce catheter occlusion, a common complication that impacts millions of IV catheters every year,” CEO Vance Clement said in a statement. “Additionally, this design enhancement would give us a differentiated product offering for the massive single lumen peripheral IV catheter market. We are very grateful for the support we’ve received from the AEDC.”
BVAD is the company’s second product behind SafeBreak Vascular, the company’s flagship product that received FDA clearance in May of 2021. BVAD is a peripheral IV catheter designed to dwell in the vein while facilitating pain-free, uncontaminated blood samples through a secondary lumen dedicated to only blood draws. Lineus Medical believes the use of BVAD will likely reduce phlebotomy costs and make inpatient hospital stays more comfortable for diabetics and other patients that require frequent blood sampling.
“This TDP funding is critical because it allows BVAD development to move forward in a meaningful way,” founder and chief technology officer Spencer Jones said in a statement. “With these funds, we will be able to validate the safety and performance of a unique design enhancement against industry-leading peripheral IV catheters.”
According to the release, the funding awarded through the TDP is non-dilutive and does not require matching funds from the company receiving the award. Royalties from sales generated from the developed technology will be collected by the AEDC in accordance with the royalty agreement reached by the AEDC and the award recipient.
