Lineus Medical in Fayetteville receives 2 patents, publishes canine research
by August 25, 2022 1:57 pm 1,020 views
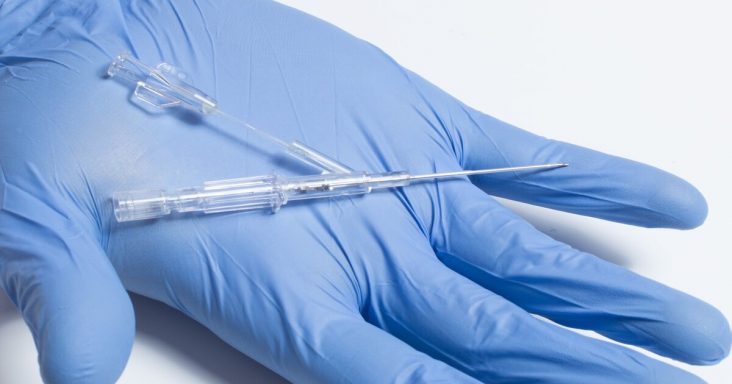
Lineus Medical's BVAD (Bifurcated Venous Access Device).
Fayetteville-based healthcare startup Lineus Medical recently announced receiving two more patents on its products and has published research in a veterinary medical journal.
The SafeBreak Vascular, a break-away medical device that helps protect peripheral IV lines, received a Japanese Utility Patent. The Bifurcated Venous Access Device (BVAD), a dual-lumen peripheral IV catheter, received a U.S. utility patent.
“The issuance of both the Japanese patent for SafeBreak Vascular and our U.S. patent for BVAD are significant milestones as we expand our patent portfolio and look to expand internationally,” said CEO Vance Clement. “These patents are critical steps in our mission to remove the pains associated with medical lines, not just in the U.S. but globally.”
Lineus Medical now has nine patents from various countries, with more patents pending.
“Japan is the second-largest medical device market in the world, so securing patent protection for SafeBreak Vascular in this market captures enormous enterprise value for our organization,” said Spencer Jones, founder and chief technology officer for Lineus Medical. “BVAD had already been granted patent protection in several international markets, and obtaining a patent in the U.S. is another invaluable addition to our portfolio. Consistent innovation is crucial for emerging companies like Lineus Medical, and protecting those ideas is paramount.”
In June 2021, Lineus Medical announced that the U.S. Food and Drug Administration (FDA) cleared for sale the SafeBreak Vascular, the startup’s first medical device.

“The majority of 2021 after we got clearance was devoted to building out the sales force and adjusting to the post-COVID selling environment. But we have a number of hospitals using SafeBreak right now, and some really key accounts we’re getting over the finish line,” Jones said.
The startup is working to receive clearance to sell the medical device in Canada, South Korea and Europe, he said. Sales of the device are expected to start in the next year in Canada and South Korea and within 18 to 24 months in Europe.
According to the news release, the breakaway device is designed to remove harmful forces from peripheral IV lines by intentionally separating when damaging levels of force are applied to the IV line. The device helps to reduce the need for an IV restart.
Jones said Lineus Medical has yet to apply for FDA clearance for the BVAD.
“We’ve been laser-focused on SafeBreak and have been devoting the majority of our resources toward its product launch, but we have been working behind the scenes to keep BVAD development moving along,” Jones said. “The issuance of the patent doesn’t impact the regulatory clearance side of things, but it’s certainly an important milestone for the product and our company as a whole.”
According to the news release, the BVAD, the startup’s second device in the works, allows for uncontaminated blood draws from a dedicated secondary lumen while allowing normal infusions through the primary lumen. The device complements the company’s goal that every patient should have one catheter and one needlestick per hospital stay and is expected to provide value and efficiency to nurses and hospitals.
Lineus Medical has eight full-time employees and multiple openings for sales positions.
“Attracting talent is so critical for growing companies,” Jones said. “It’s been exciting to see our headcount more than double over the past year.”
CANINE STUDY
The Journal of American Veterinary Medical Association recently published the results of a study that included 367 dogs who were admitted to a Colorado veterinary hospital and used Lineus Medical’s peripheral IV breakaway device to reduce IV complications.
The randomized controlled trial performed at Colorado State University Veterinary Teaching Hospital documented the impact that a new class of peripheral IV breakaway devices had on IV complications in dogs admitted to the hospital. Lineus Medical sponsored the study to document the impact that SafeBreak has on IV complication rates compared to the standard of care.
The results of the study showed that the use of SafeBreak Vascular Vet, Lineus Medical’s breakaway device, reduced IV complications by 65%. The results also showed that the device may limit patient discomfort, owner (client) expense and staff time managing IV complications. The SafeBreak Vascular Vet used in the study functions similarly to the SafeBreak Vascular for humans.
According to the study, the device has been proven to reduce IV complications in small and large animals and reduced IV-line breakage by 92%, dislodgement by 80%, phlebitis by 86% and occlusion by 100%.
“Reducing peripheral IV complications is imperative as it ensures that the patient receives all prescribed treatments in a timely manner, but it also may reduce the risk for venous depletion, reduce client costs and minimize pain for the dog,” said Sydney Simpson, co-author of the study.
Dr. Kristin Zersen, principal investigator and senior study author, emphasized the 65% decline in overall IV complications and noted the cost of replacing an IV was about $60, compared to the $6.90 cost to replace the SafeBreak device.
“This reduction in complications equates to a significant cost savings to the client,” Zersen said. “Just as important, it reduces the need for IV replacement, which can cause pain and anxiety for our patients.”
Jones highlighted the significance of the study results.
“In veterinary medicine, the way procedures and supplies are billed to the pet owner is very straightforward compared to a human hospital,” Jones said. “This allowed us to clearly show that reducing IV complications with SafeBreak delivers a direct clinical and financial benefit for patients, clients and care providers.”
