Fayetteville startup Lineus Medical cleared by FDA to sell its first medical device
by June 1, 2021 2:50 pm 2,810 views
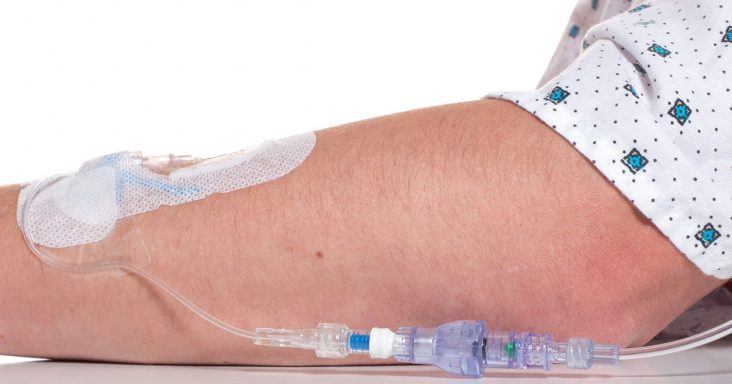
A SafeBreak Vascular installed in an IV line.
Fayetteville-based healthcare technology startup Lineus Medical announced Tuesday (June 1) that the U.S. Food and Drug Administration (FDA) has cleared for sale the startup’s first medical device, SafeBreak Vascular. According to a news release, it’s the first Force-Activated Separation Device cleared for sale in the United States through the FDA’s De Novo review process.
“Achieving FDA De Novo Designation as a Class II device is no doubt the biggest milestone to date for our company,” said Spencer Jones, founder and chief technology officer of Lineus Medical. “We’re ecstatic about reaching this inflection point and eager to start executing on our product launch plan. It took an incredible amount of perseverance, a lot of investment and a talented team to overcome the challenges we faced along the way.”
Vance Clement, CEO of Lineus Medical, said, “we want to thank our investors for their continued support, especially through the COVID-19 pandemic. A randomized clinical trial completed in 2021 showed the device worked as advertised, eliminating spills and saving nurses a great deal of time. The study most importantly showed a 44% reduction of peripheral IV complications requiring an IV restart in the SafeBreak Vascular group when compared to the control group. Now we are excited to get the product into the hands of clinicians everywhere so that we can start to positively impact a lot more patient’s lives.”
SafeBreak Vascular is distributed on behalf of Lineus Medical by MediCore of Nashville, Tenn., along with multiple other independent sub-distributors across the United States. MediCore distributes other vascular access products to more than 1,100 U.S. hospitals.

Jones founded Lineus Medical, formerly LineGard Med, in May 2015 to develop and bring SafeBreak Vascular to market.
According to the news release, the device reduces mechanical complications requiring IV restarts in peripheral IV lines when tension greater than 4 pounds of force is placed on the line. Tugs or pulls on IV lines are typical and part of a patient’s time in the hospital. The existing method for securing IV catheters includes using adhesive dressings and securement devices. These methods focus on securing the line to the patient’s skin and tend to fail when the IV tubing is subjected to large pull forces. SafeBreak Vascular is unique from those methods and part of a new class of devices called Force-Activated Separation Devices. These devices separate to remove the damaging force from the IV line and prevent the loss of blood and medication.
When the device separates, a valve closes at each end of it. The IV is left intact on the patient, and the valves close to prevent the patient from bleeding and stops the IV pump from pumping medicine on the floor or in the patient’s bed. When the pump’s fluid flow stops, an alarm is triggered to alert medical staff. A separated SafeBreak Vascular can be discarded and a new one installed in the line in a few minutes. The patient’s medical treatment can resume by restarting the IV infusion without needing a new catheter or additional needle sticks.
IV restarts are estimated to cost the U.S. healthcare system more than $5 billion annually. The cost of a restart is about $50 each. More than 235 million IV lines are placed annually in the United States, and an average of 46% fail before their intended end of use, the release shows.
