Springdale company’s COVID antibody test approved by FDA
by May 26, 2021 7:25 am 1,693 views
Springdale-based NOWDiagnostics Inc. has received Emergency Use Authorization (EUA) to use its proprietary AdexusDx product line for COVID-19 antibody testing in moderate/complex laboratory settings and at the point of care in the U.S.
The company submitted the application to the U.S. Food and Drug Administration (FDA) on May 29, 2020.
According to a news release Wednesday (May 26), C19 Development LLC, a wholly-owned subsidiary of NOWDiagnostics, will begin offering the test for use across a variety of CLIA-waived healthcare settings including pharmacies, clinics and hospital emergency rooms. Studies are ongoing to make the patented technology available for over-the-counter detection of SARS-CoV-2 antibodies.
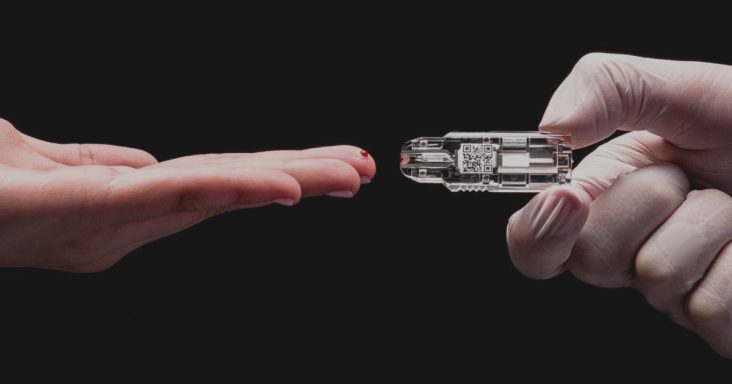
“The AdexusDx COVID-19 test is literally a lab at the tip of your finger, specifically designed to make diagnostic testing possible at home,” NOWDiagnostics CEO Kevin Clark said in a statement. “This EUA approval is the first step in making that a reality. The AdexusDx test uses the same platform as our other FDA-cleared and/or CE marked, next-generation tests which are affordable, portable, and deliver laboratory-quality results in minutes without any additional supplies.
“We believe antibody testing is essential to America’s recovery from the COVID-19 pandemic, especially with the ongoing rollout of vaccines, and we’re proud to be responding to the urgent need for a reliable COVID-19 antibody test that targets the spike protein with our made-in-the-U.S.A. product.”
 NOWDiagnostics’ AdexusDx product line uses a drop of blood to test for a variety of conditions, illnesses and diseases with results in a matter of minutes. The benefit is the elimination of the need to send tests to off-site laboratories, which decreases the waiting period to determine results.
NOWDiagnostics’ AdexusDx product line uses a drop of blood to test for a variety of conditions, illnesses and diseases with results in a matter of minutes. The benefit is the elimination of the need to send tests to off-site laboratories, which decreases the waiting period to determine results.
The company said its products have the potential to decrease the waiting period to determine test results by days.
Congressman Steve Womack, R-Rogers, along with U.S. Senators John Boozman and Tom Cotton issued the following statement regarding the approval:
“Thanks to the incredible work of NOWDiagnostics, the only U.S.-made antibody test of its kind was developed and manufactured here in Springdale. And while every Arkansan should be proud of our state’s contribution to America’s health, unfortunately, slow FDA approval prevented its earlier release. We will work together to ensure the FDA prioritizes a more efficient review process.”
