NOWDiagnotics seeks participants for usability study
by October 27, 2020 4:19 pm 1,071 views
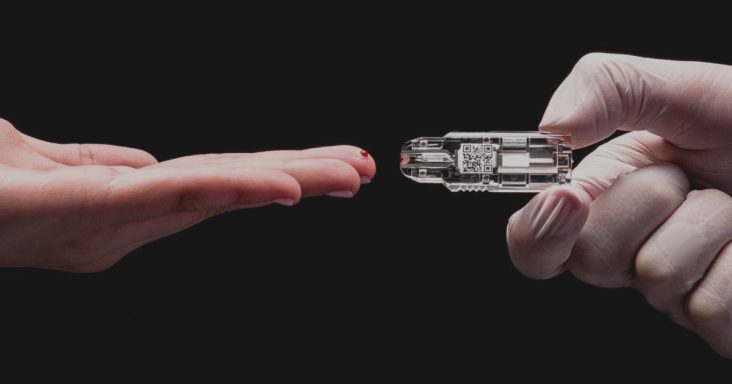
Springdale-based NOWDiagnostics is seeking 165 area participants to help the med-tech company with future trials. The company is asking subjects to be part of two usability studies that provide feedback on ease of use and clarity of directions associated with administering diagnostic tests.
Beth Cobb, director of operations for NOWDiagnostics, said usability studies do not require the subjects to get the test, but is a study to evaluate if they could easily understand how to give the tests to themselves or administer it to someone else. She said adult individuals, those with kids and teenagers, will be enlisted to provide feedback in the upcoming study. Cobb said the company relies on feedback from users to submit the best information to the U.S. Food and Drug Administration (FDA).
Cobb said recruiting subjects in the region has had its challenges because they often do not understand what they entail. She said the knowledge and insights the company has been able to glean have been invaluable to the company’s work. She said because the company’s goal is to design easy-to-use, portable diagnostic tests, it’s imperative the public understands how to administer the test correctly, with little to no margin for error.
NOWDiagnostics focuses on the development and dissemination of portable diagnostics that can be performed with quick and accurate results on the spot, whether it’s detecting pregnancy, HIV antibodies or antibodies for COVID-19.
Kevin Clark, CEO of NOWDiagnostics, said recently at the NWA Technology Summit the company’s test can detect pregnancy as soon as 48 hours, several days sooner than the standard urine test. Clark said the self-use diagnostic test is being sold over-the-counter in Europe. The test uses a single drop of blood to detect pregnancy and is approved for use in doctor’s offices and clinics in the U.S.
The company has also designed a diagnostic test that uses a drop of blood to test for COVID-19 antibodies. Trials for the diagnostic test took place in Arizona, Florida and Tennessee earlier this year and the results were submitted to the FDA in May. The company was asked to repeat a few experiments and they resubmitted the additional data and await approval for their testing protocol for use by clinics and other U.S. healthcare venues.
The company’s diagnostic COVID antibody test has been approved for laboratory use in Europe. Cobb said the company is selling antibody tests abroad including in the Middle East and in South America.
Cobb said the company is also collecting data to help them develop a salvia-based test protocol for antibodies and ultimately antigens to COVID-19. That data collection is ongoing in Northwest Arkansas with the help of Mercy Research in Rogers and St. Louis and MANA Health. Cobb said the providers are asking patients who actively test positive for COVID-19 if they want to take part in the NOWDiagnostics research around antibody and antigen tests that the company hopes to develop with a saliva-based protocol.
Clark said there is a need for more diagnostic testing. He said COVID-19 pushed the need ahead by years. He said the company is looking to make testing easier and bring it closer to the consumer. He said 13 billion diagnostic tests are performed annually in the U.S. and having a protocol that allows anyone to run the test and get lab-quality results at a cost of less than a copay could revolutionize the industry.
Clark can foresee a time when parents might buy a strep kit off the shelf at retail and quickly test their kid for strep bacteria from one drop of saliva. He said parents could have the results in minutes and save a trip to the doctor. He said the turn-key tests are so small he could fit 5,000 of them into a backpack. The entire test is contained in one cartridge.
“It’s like having a lab at your fingertips,” he said. “It’s portable and provides reliable results in 15 minutes.”
Anyone who wants additional information about taking part in the upcoming usability study or other research can email the company at [email protected] or phone 844-966-4530.
