NOWDiagnostics, BARDA partner to develop rapid COVID-19 antibody test for use point-of-care and at-home
by August 31, 2020 3:34 pm 977 views
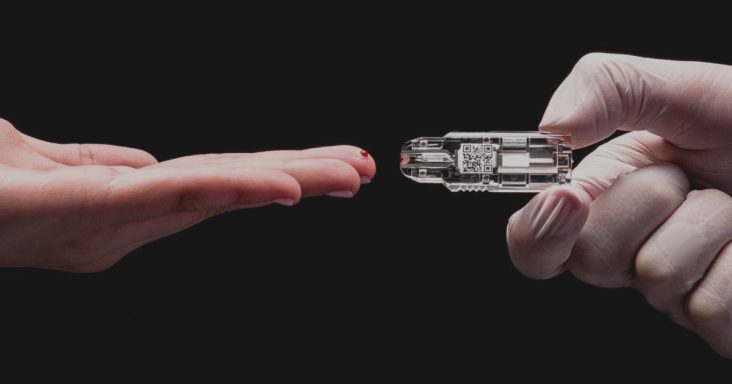
Springdale-based NOWDiagnostics Inc. said Monday (Aug. 31) it is working with U.S. government agency Biomedical Advanced Research and Development Authority (BARDA) to develop a serological test for COVID-19 antibodies that can be used across a variety of healthcare settings from clinics to hospital emergency rooms — and ultimately by consumers for at-home use.
The COVID-19 rapid antibody test, using NOWDiagnostics’ proprietary AdexusDx product line, requires no additional materials (reagents and buffers), equipment, processing or refrigeration, according to a news release.
NowDiagnostics’ AdexusDx product line uses a drop of blood to test for a variety of conditions, illnesses and diseases with results in a matter of minutes. The benefit is the elimination of the need to send tests to off-site laboratories, which decreases the waiting period to determine results.
No matter where it is conducted, the AdexusDx COVID-19 test is designed to deliver lab-quality results in 15 minutes detecting the presence of COVID-19 antibodies in individuals who have been exposed to the virus. That can include those who have been recently or previously infected with COVID-19, regardless of whether they presented with severe, moderate, mild or no symptoms.
Availability of point-of-care (POC) and over-the-counter (OTC) tests would allow individuals to be screened easily and quickly, yielding test results in minutes instead of days.
NOWDiagnostics submitted an application to the FDA for Emergency Use Authorization (EUA) of the test on May 29, 2020. That application is still pending.
The project is funded in part with federal funds from the Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response and BARDA’s Division of Research Innovation and Ventures.
BARDA’s support for the project includes funding of $695,500 of the project costs and technical support required for NOWDiagnostics to request the EUA.
