Springdale company’s COVID antibody test approved in EU
by July 28, 2020 9:46 am 1,858 views
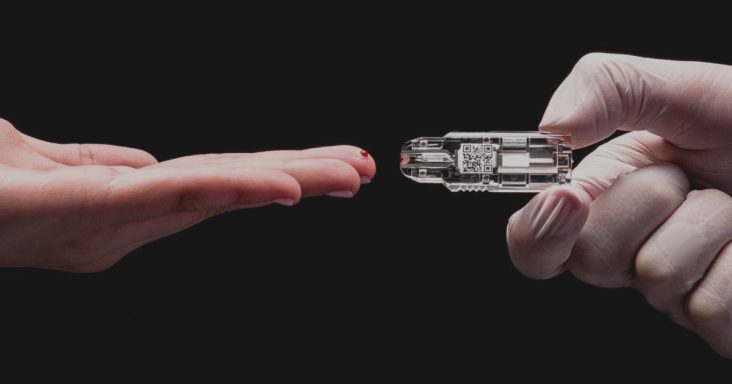
Springdale-based NOWDiagnostics Inc. has received approval to use its proprietary AdexusDx product line for COVID-19 antibody testing in moderate/complex laboratory settings across 28 countries in the European Union (EU). The company submitted its application for the antibody test in mid-June, according to a Tuesday (July 28) news release.
C19 Development LLC, a wholly owned subsidiary of NOWDiagnostics, will begin offering the AdexusDx test in a variety of healthcare settings in the EU — from clinics to hospital emergency rooms — while launching clinical trials of the test for use at point-of-care and over-the-counter.
NowDiagnostics’ AdexusDx product line uses a drop of blood to test for a variety of conditions, illnesses and diseases with results in a matter of minutes. The benefit is the elimination of the need to send tests to off-site laboratories, which decreases the waiting period to determine results.
In the U.S., NOWDiagnostics submitted an application to the FDA for Emergency Use Authorization (EUA) of the test on May 29, 2020. That application is still pending.
“The AdexusDx COVID-19 test is literally a lab at the tip of your finger, specifically designed to make diagnostic testing possible anywhere,” NOWDiagnostics CEO Kevin Clark said in a statement. “While this CE [Conformité Européene] mark permits our test to be used in laboratory settings, we expect that after completing clinical trials and obtaining further regulatory approvals, our test will be available to everyone. CE mark of our test is the first step in making that a reality.”
NOWDiagnostics was founded in 2013 and opened its 22,000-square-foot manufacturing operation in 2014 in Springdale. The company, according to its website, sells hCG (pregnancy) tests in the United States and Europe, and three cardiac (heart attack) tests and three toxicology tests in Europe. Additional tests are in the development pipeline, including sexually transmitted diseases, food intolerances, common infectious diseases, and a variety of screening tests.
